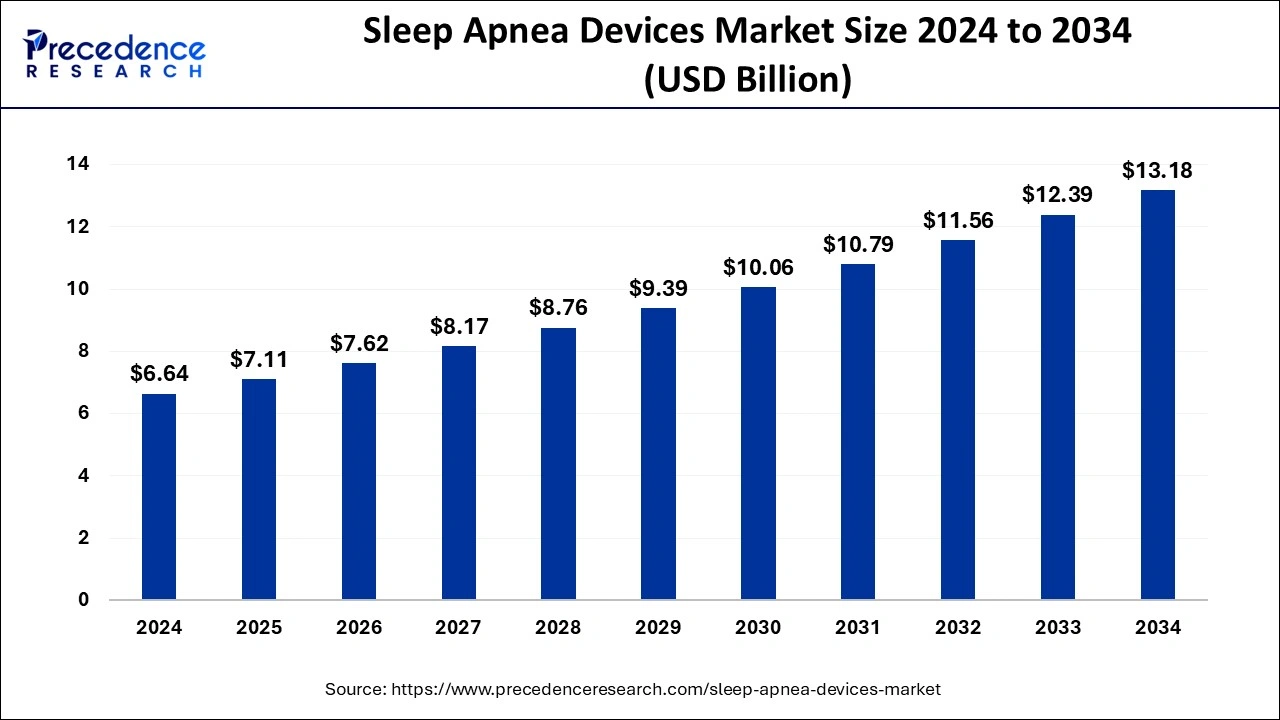
The global sleep apnea devices market size is estimated at USD 6.64 billion in 2024 and it is expected to cross USD 13.18 billion by 2034 with a notable CAGR of 7%.
Get Sample Copy of Report@ https://www.precedenceresearch.com/sample/1053
Key Insights
- North America emerged as the market leader in 2024, capturing a 49% share.
- Asia Pacific is anticipated to witness the highest CAGR in the coming years.
- The therapeutic devices segment led the market in terms of product share.
Market Drivers
The sleep apnea devices market is driven by the rising prevalence of sleep disorders, particularly obstructive sleep apnea (OSA), which has been linked to lifestyle changes, obesity, and aging populations. Growing awareness about the health risks associated with untreated sleep apnea, such as cardiovascular diseases and diabetes, is increasing the demand for effective treatment options.
Technological advancements, including compact and user-friendly CPAP devices, have further fueled market growth. Additionally, favorable reimbursement policies and increasing healthcare expenditures in developed economies are supporting the adoption of sleep apnea devices.
Market Opportunities
The market presents significant opportunities due to the increasing focus on home healthcare and portable sleep apnea devices. The growing integration of artificial intelligence and cloud-based monitoring in sleep apnea devices enhances patient compliance and treatment outcomes.
Emerging markets, particularly in Asia Pacific and Latin America, offer lucrative opportunities due to improving healthcare infrastructure and rising disposable incomes. Furthermore, ongoing research and development efforts to develop alternative therapies, such as neurostimulation devices, are expected to create new growth avenues.
Market Challenges
Despite the positive outlook, the market faces challenges such as the high cost of sleep apnea devices, which can limit accessibility, particularly in low-income regions. Patient adherence to continuous positive airway pressure (CPAP) therapy remains a concern, as many users find the devices uncomfortable or difficult to use.
Additionally, the lack of awareness and underdiagnosis of sleep apnea in many developing regions hinder market growth. Stringent regulatory requirements and lengthy approval processes for new devices also pose obstacles for market players.
Regional Insights
North America holds the largest share of the sleep apnea devices market due to a high prevalence of sleep disorders, well-established healthcare infrastructure, and strong reimbursement policies. Europe follows closely, supported by increasing awareness and advancements in sleep apnea treatment.
The Asia Pacific region is expected to witness the fastest growth due to rising healthcare investments, a growing geriatric population, and increasing lifestyle-related disorders. Latin America and the Middle East & Africa regions are also experiencing steady growth, driven by improving healthcare access and growing awareness of sleep-related disorders.
Sleep Apnea Devices Market Companies
- GE Healthcare
- BMC Medical
- Philips Healthcare
- Weinmann Medical Devices
- CadwellLaboratorie
- Compumedics
- Curative Medical
- Teleflex
- CareFusion Corporation
- Fisher &Paykel Healthcare
- ResMed
- SOMNOmedics
- Invacare Corporation
- Natus Medical
Latest Announcement by Industry Leaders
- John Lopos, CEO of the National Sleep Foundation, commented that AI can expand sleep-tracking capabilities, enabling personalized information to be shared with users and empowering them to make small behavioral changes for the better.
Recent Developments
- In September 2024, the U.S. Food and Drug Administration approved Apple Watch’s sleep apnea detection feature. The device detects sleep apnea by identifying breathing disturbances using wrist movements.
- In August 2024, Inspire Medical Systems announced that it received the U.S. FDA for its Inspire V obstructive sleep apnea neurostimulator therapy device. The device contains a sensing function and a software-based platform for adding new features and will fully launch in 2025.
Segments Covered in the Report
By Product Type
- Diagnostic Devices
- Respiratory Polygraphs
- Actigraphs
- Polysomnography (PSG) Device
- Pulse Oximeters
- Therapeutic Devices
- Nasal Devices
- Positive Airway Pressure (PAP) Devices
- Oral Devices
- Chin Straps
By End-User
- Home Care Setting
- Sleep Laboratories & Hospitals
- Others
By Regional Outlook
- North America
- U.S.
- Canada
- Europe
- U.K.
- Germany
- France
- Asia Pacific
- China
- India
- Japan
- South Korea
- Middle East & Africa
- Latin America
Ready for more? Dive into the full experience on our website@ https://www.precedenceresearch.com/
- Scleral Lens Market Size to Gain USD 982.68 Bn by 2034 - April 11, 2025
- Water for Injection Market Size to Attain USD 87.39 Billion by 2034 - April 10, 2025
- ELISpot and FluoroSpot Assay Market Size to Reach USD 576.59 Million by 2034 - April 10, 2025