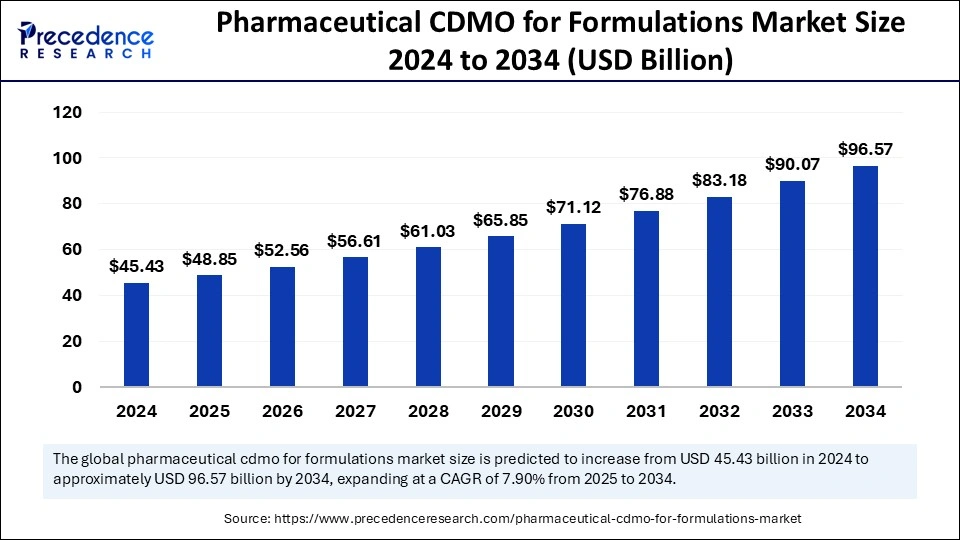
The global pharmaceutical CDMO for formulations market size is expected to attain around USD 96.57 billion by 2034, increasing from USD 45.43 billion in 2024, with a CAGR of 7.90%.
Get a Free Sample Copy of the Report@ https://www.precedenceresearch.com/sample/5785
Pharmaceutical CDMO for Formulations Market Key Takeaways
-
The Asia Pacific region emerged as the market leader in 2024, claiming a 42% share.
-
Europe is poised for strong expansion with an 8.40% compound annual growth rate.
-
Oral solid dosage forms dominated the market with a substantial 40% share.
-
The injectables segment is on a growth path, projected to rise at 8.31% CAGR.
-
Oncology stood out as the leading therapeutic focus, securing 23% market contribution.
-
Infectious disease applications are expanding steadily at 8.02% CAGR.
-
Pharmaceutical companies accounted for the majority of demand, holding 55% market share.
-
Biopharmaceutical companies are catching up quickly, forecasted to grow at 8.17% CAGR.
AI Revolutionizing Pharmaceutical CDMOs for Formulations
Smart Formulation Development
Artificial Intelligence is redefining how pharmaceutical CDMOs approach drug formulation. By using advanced algorithms and predictive modeling, AI can simulate countless formulation combinations, optimize ingredient ratios, and ensure long-term stability—all before physical trials begin. This not only saves time but also cuts R&D costs dramatically while boosting formulation precision and success rates.
Precision Manufacturing & Smarter Operations
In manufacturing, AI steps in as the digital brain—monitoring operations in real time, predicting equipment failures, and maintaining strict quality standards. From automated batch processing to adaptive quality control, AI helps CDMOs improve product consistency, reduce downtime, and scale up with confidence. Plus, with AI-enhanced supply chain forecasting, companies can meet market demands faster and more efficiently than ever.
Pharmaceutical CDMO for Formulations Market Growth Factors
1. Rising Demand for Outsourced Drug Development
Pharmaceutical and biotech companies are increasingly outsourcing formulation and manufacturing processes to CDMOs to reduce costs, accelerate timelines, and focus on core competencies like R&D and marketing. This trend is especially strong among small to mid-sized firms with limited internal infrastructure.
2. Surge in Complex and Personalized Therapies
As the industry shifts toward more complex formulations—including biologics, injectables, and personalized medicines—CDMOs with advanced formulation expertise and adaptable technologies are in high demand. Their ability to manage sophisticated development processes makes them vital partners.
3. Expanding Biopharmaceutical Sector
The biopharma boom has significantly boosted the need for CDMOs with capabilities in sterile manufacturing, high-potency drugs, and large molecule formulations. Biopharma companies rely heavily on specialized CDMOs to manage strict regulatory demands and scale-up requirements.
4. Globalization and Market Expansion
Pharmaceutical companies are expanding into emerging markets, increasing the need for regionally compliant formulations. CDMOs with global manufacturing and regulatory capabilities are well-positioned to support localized product development and distribution.
5. Technological Advancements and AI Integration
The adoption of cutting-edge technologies such as AI, machine learning, and digital twins enhances formulation optimization, predictive analytics, and operational efficiency. These innovations help CDMOs offer faster, more accurate, and cost-effective solutions.
6. Stringent Regulatory Standards and Quality Requirements
Meeting the rising regulatory standards for drug quality, safety, and efficacy is challenging. CDMOs that invest in robust quality systems, GMP compliance, and regulatory support become preferred partners for pharmaceutical companies.
Market Scope
| Report Coverage | Details |
| Market Size by 2034 | USD 96.57 Billion |
| Market Size in 2025 | USD 48.85 Billion |
| Market Size in 2024 | USD 45.43 Billion |
| Market Growth Rate from 2025 to 2034 | CAGR of 7.90% |
| Dominating Region | Asia Pacific |
| Fastest Growing Region | Europe |
| Base Year | 2024 |
| Forecast Period | 2025 to 2034 |
| Segments Covered | Dosage Form, Therapeutic Area, End-User, and Regions. |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Market Dynamics
Key Driving Forces
The market is driven by increasing R&D expenditure, the complexity of drug formulations, and the pressure to reduce drug development timelines. Demand for advanced oral and injectable formulations is especially fueling CDMO growth.
Emerging Opportunities
Emerging markets, biologics, and advanced drug delivery systems represent significant areas of opportunity. The push toward sustainable and continuous manufacturing also opens new avenues for innovation in CDMO services.
Persistent Challenges
Maintaining consistent quality, navigating regulatory environments, and building robust manufacturing capacities are key challenges. CDMOs must also manage scalability while ensuring data integrity and supply chain reliability.
Regional Perspective
Asia Pacific holds the largest market share due to affordability and scalability. Europe is expected to expand steadily thanks to innovation-friendly policies, and North America benefits from strong investments and regulatory guidance fostering CDMO partnerships.
Pharmaceutical CDMO for Formulations Market Companies

- Lonza
- Thermo Fisher Scientific, Inc.
- Recipharm AB
- Laboratory Corporation of America Holdings (LabCorp)
- Catalent, Inc.
- WuXi AppTec, Inc.
- Piramal Pharma Solutions
- Siegfried Holding AG
- CordenPharma International
- Cambrex Corporation
- Bushu Pharmaceuticals Ltd.
- Nipro Corporation
- EuroAPI
- Hovione
- Curia
Latest Announcements by Industry Leaders
- In November 2024, John Rim, Samsung Biologics chief executive, stated that the company feels honored to improve its partnership with European pharmaceutical companies to jointly deliver high-quality biopharmaceutical treatment to patients. The strategic global partnerships invest in its technologies and manufacturing capabilities. We aim to provide the highest quality services at every stage and deepen our trusted partnerships.
Recent Developments
- In October 2023, Cambrex Corporation, centered in High Point, North Carolina (U.S.), finished its expansion project worth USD 38 million. The facility improvement delivered cutting-edge analytical laboratories as well as chemical development laboratories, new clinical production suites, and three work centers with 2,000 L reactors for small-scale commercial manufacturing.
Segments Covered in the Report
By Dosage Form
- Oral Solids
- Oral Liquids
- Injectables
- Topicals
- Inhalation Products
- Transdermal And Patches
- Others
By Therapeutic Area
- Oncology
- Cardiology
- Central Nervous System
- Gastroenterology
- Infectious Diseases
- Endocrinology (Diabetes, Hormonal Therapy)
By End User
- Pharmaceutical Companies
- Biopharmaceutical Companies
- Others
By Region
- North America
- Asia Pacific
- Europe
- Latin America
- Middle East and Africa
Also Read: CRISPR-Based Gene Editing Market
Ready for more? Dive into the full experience on our website@ https://www.precedenceresearch.com/
- Myopia Treatment Devices Market Size to Reach USD 38.51 Billion by 2034, Growing at a CAGR of 7.86% - September 1, 2025
- Pill Timer Market Size to Hit $2.94 Bn by 2034 | 9.02% CAGR Growth Forecast - August 12, 2025
- Blood Glucose Monitoring Market Size to Worth USD 25.53 Billion by 2034 - August 11, 2025