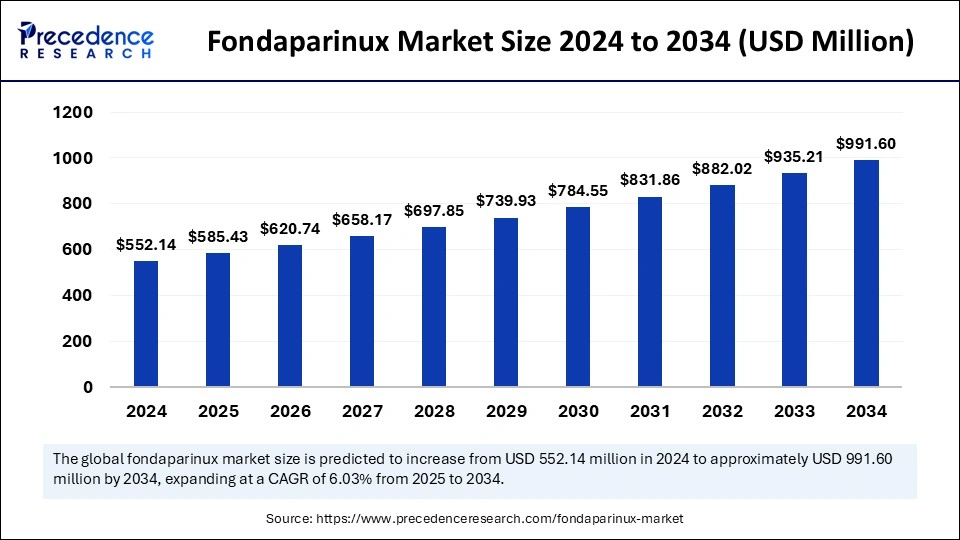
The global fondaparinux market size was valued at USD 552.14 million in 2024 and is expected to attain around USD 991.60 million by 2034, growing at a CAGR of 6.03%.
Get a Free Sample Copy of the Report@ https://www.precedenceresearch.com/sample/5747
Key Insights
-
North America led the fondaparinux industry with a 38% market share in 2024.
-
Asia Pacific is anticipated to experience a 7.51% CAGR growth.
-
Deep vein thrombosis (DVT) remained the dominant type, holding a 61% share.
-
The pulmonary embolism (PE) segment is projected to grow at a CAGR of 6.7%.
-
The generic fondaparinux segment accounted for the largest share (53%) in 2024.
-
The branded segment is set to expand at a CAGR of 6.1% over the forecast period.
-
Retail pharmacies held a 56% market share in 2024.
-
Online pharmacies are expected to grow significantly at a CAGR of 11.2%.
Fondaparinux: Overview
What is Fondaparinux?
Fondaparinux is a synthetic anticoagulant (blood thinner) that belongs to the class of selective Factor Xa inhibitors. It is primarily used to prevent and treat blood clots, including deep vein thrombosis (DVT) and pulmonary embolism (PE).
Mechanism of Action
-
Fondaparinux selectively binds to antithrombin III, enhancing its ability to inhibit Factor Xa.
-
By blocking Factor Xa, it prevents thrombin formation, reducing the risk of blood clot development.
-
Unlike heparin, fondaparinux does not have a direct effect on thrombin (Factor IIa).
Medical Uses
-
Prevention of Blood Clots
-
Used in patients undergoing orthopedic surgeries (hip fracture, knee, or hip replacement).
-
Reduces the risk of venous thromboembolism (VTE) in hospitalized patients.
-
-
Treatment of Deep Vein Thrombosis (DVT)
-
Used alone or in combination with other anticoagulants.
-
-
Treatment of Pulmonary Embolism (PE)
-
Often used as an initial treatment before transitioning to warfarin or direct oral anticoagulants (DOACs).
-
-
Acute Coronary Syndrome (ACS)
-
Used in some cases of unstable angina or myocardial infarction (heart attack).
-
Dosage & Administration
-
Administered subcutaneously (under the skin).
-
Dosage depends on the patient’s body weight and the condition being treated.
-
Usually given once daily due to its long half-life.
Advantages of Fondaparinux
-
Lower risk of heparin-induced thrombocytopenia (HIT) compared to heparin.
-
Predictable dosing without routine monitoring.
-
Long half-life allows once-daily dosing.
-
No direct impact on platelet function.
Limitations & Side Effects
-
Bleeding risk (like all anticoagulants).
-
No specific reversal agent (unlike heparin, which can be reversed with protamine sulfate).
-
Kidney function concerns – Contraindicated in patients with severe renal impairment (CrCl < 30 mL/min).
Role of AI in the Fondaparinux Market
Artificial intelligence (AI) is playing a transformative role in the fondaparinux market by enhancing drug development, optimizing supply chains, improving patient care, and driving market insights. Here are the key areas where AI is making an impact:
1. Drug Discovery & Development
-
AI accelerates the identification of novel anticoagulants and enhances research on fondaparinux derivatives.
-
Machine learning models analyze molecular interactions to improve drug formulations and effectiveness.
-
AI-driven simulations help predict drug behavior, reducing reliance on expensive and time-consuming clinical trials.
2. Personalized Medicine & Dosage Optimization
-
AI analyzes patient data, including genetic, lifestyle, and clinical factors, to personalize fondaparinux dosage and reduce adverse effects.
-
Predictive algorithms assess the risk of bleeding and thrombosis, ensuring safer anticoagulant therapy.
3. Market Analysis & Forecasting
-
AI-powered tools analyze global fondaparinux demand, market trends, and competition, helping pharmaceutical companies make data-driven decisions.
-
Real-time monitoring of sales and prescription patterns enables businesses to optimize pricing and distribution strategies.
-
AI models predict market growth, such as the expected rise of online pharmacy sales and the growing demand in Asia Pacific.
4. Supply Chain & Distribution Management
-
AI enhances supply chain efficiency by predicting demand fluctuations and optimizing inventory levels.
-
Automated logistics and AI-driven tracking ensure timely delivery of fondaparinux to hospitals and pharmacies.
-
AI detects counterfeit drugs in the supply chain by analyzing packaging, batch numbers, and distribution patterns.
5. Patient Monitoring & Adherence
-
AI-powered mobile apps and wearable devices monitor patient adherence to fondaparinux therapy, reducing the risk of complications.
-
Chatbots and virtual assistants provide medication reminders, answer patient queries, and improve engagement.
-
AI-driven electronic health records (EHRs) flag potential drug interactions and adverse effects in real-time.
6. Regulatory Compliance & Pharmacovigilance
-
AI automates regulatory reporting and ensures fondaparinux meets safety and compliance standards.
-
Machine learning models detect adverse drug reactions (ADRs) by analyzing vast amounts of patient data from clinical trials and post-market surveillance.
-
AI-driven natural language processing (NLP) helps pharmaceutical companies stay updated with regulatory changes worldwide.
Future Potential of AI in the Fondaparinux Market
-
Advanced AI models could further refine real-time risk assessment for patients on fondaparinux.
-
Integration of AI with blockchain could improve drug traceability and transparency.
-
AI-driven predictive analytics may help prevent shortages and optimize production strategies.
Market Scope
| Report Coverage | Details |
| Market Size by 2034 | USD 991.60 Million |
| Market Size in 2025 | USD 585.43 Million |
| Market Size in 2024 | USD 552.14 Million |
| Market Growth Rate from 2025 to 2034 | CAGR of 6.03% |
| Dominated Region | North America |
| Fastest Growing Market | Asia Pacific |
| Base Year | 2024 |
| Forecast Period | 2025 to 2034 |
| Segments Covered | Type, Product, Distribution Channel, and Regions. |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America and Middle East & Africa |
Market Dynamics
Market Drivers
Several factors are fueling the growth of the fondaparinux market. The rising prevalence of conditions requiring anticoagulation therapy, such as deep vein thrombosis, pulmonary embolism, and acute coronary syndrome, has significantly increased fondaparinux prescriptions. The pharmaceutical industry’s investment in AI and big data analytics is enhancing drug discovery and improving treatment protocols. The push for generic drug manufacturing has made fondaparinux more affordable and accessible to a larger patient base.
Opportunities
The fondaparinux market offers substantial opportunities, particularly in regions with expanding healthcare access and government-led initiatives to prevent thromboembolic diseases. Advances in AI-powered predictive analytics are expected to enhance personalized medicine, improving treatment outcomes for anticoagulant therapies. The rise of telemedicine and online pharmacies has made it easier for patients to obtain prescriptions and medications, increasing fondaparinux sales. Additionally, research into safer anticoagulant formulations may open new avenues for product development.
Challenges
The market for fondaparinux faces notable challenges, particularly regarding its side effect profile and the absence of a direct antidote for overdose management. Competitive pressure from alternative anticoagulants, such as warfarin and newer direct oral anticoagulants (DOACs), may limit its market share. Regulatory restrictions and complex approval processes for new formulations add to the hurdles faced by pharmaceutical companies in this sector. Price fluctuations and reimbursement issues in some regions also impact accessibility.
Regional Insights
North America continues to dominate the fondaparinux market, supported by high awareness, a well-developed healthcare infrastructure, and strong pharmaceutical research. Europe follows closely, with an emphasis on cardiovascular disease prevention and extensive insurance coverage for anticoagulant therapies. The Asia Pacific region is expected to grow rapidly due to increasing healthcare expenditure, a rising aging population, and improved access to medical services. Latin America, the Middle East, and Africa are also witnessing gradual growth, driven by improving healthcare systems and greater focus on preventive care initiatives.
Fondaparinux Market Companies

- Abbott Laboratories Inc.
- Alchemia Limited
- Apotex Inc.
- GSK plc
- Lupin Pharmaceuticals, Inc
- ScinoPharm Taiwan Ltd
- Dr. Reddy’s Laboratories Ltd.
- Bayer Healthcare AG
- GlaxoSmithKline
- Boehringer Ingelheim
- Sanofi
- WisMed
- Kaifeng
- Mylan
Recent Developments
- In October 2024, Pfizer collaborated with Biocon Biologics to create a biosimilar of fondaparinux, which focuses on emerging market opportunities. The partnership works to improve markets’ access with affordable product development and optimized distribution networks.
- In December 2024, Sanofi introduced a next-generation fondaparinux injection to select European markets to boost its anti-coagulant drugs and meet rising patient needs for better therapeutic alternatives.
- In December 2024, the U.S. Food and Drug Administration approved Arixtra (fondaparinux) from Mylan for treating venous thromboembolism in children starting at 1 year of age and weighing at least 10 kg. The new product establishment added valuable items to the company’s product range.
Segments Covered in the Report
By Type
- Deep Vein Thrombosis
- Pulmonary Embolism
By Product
- Branded
- Generic
By Distribution Channel
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
By Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa
Also Read: Glycated Albumin Assay Market
Ready for more? Dive into the full experience on our website@ https://www.precedenceresearch.com/
- Raman Spectroscopy Market Size to Surge USD 2.88 Bn by 2034 - March 31, 2025
- Vaccine Storage and Packaging Market Size to Attain USD 37.39 Bn by 2034 - March 31, 2025
- Fondaparinux Market Size to Attain USD 991.60 Mn by 2034 - March 28, 2025