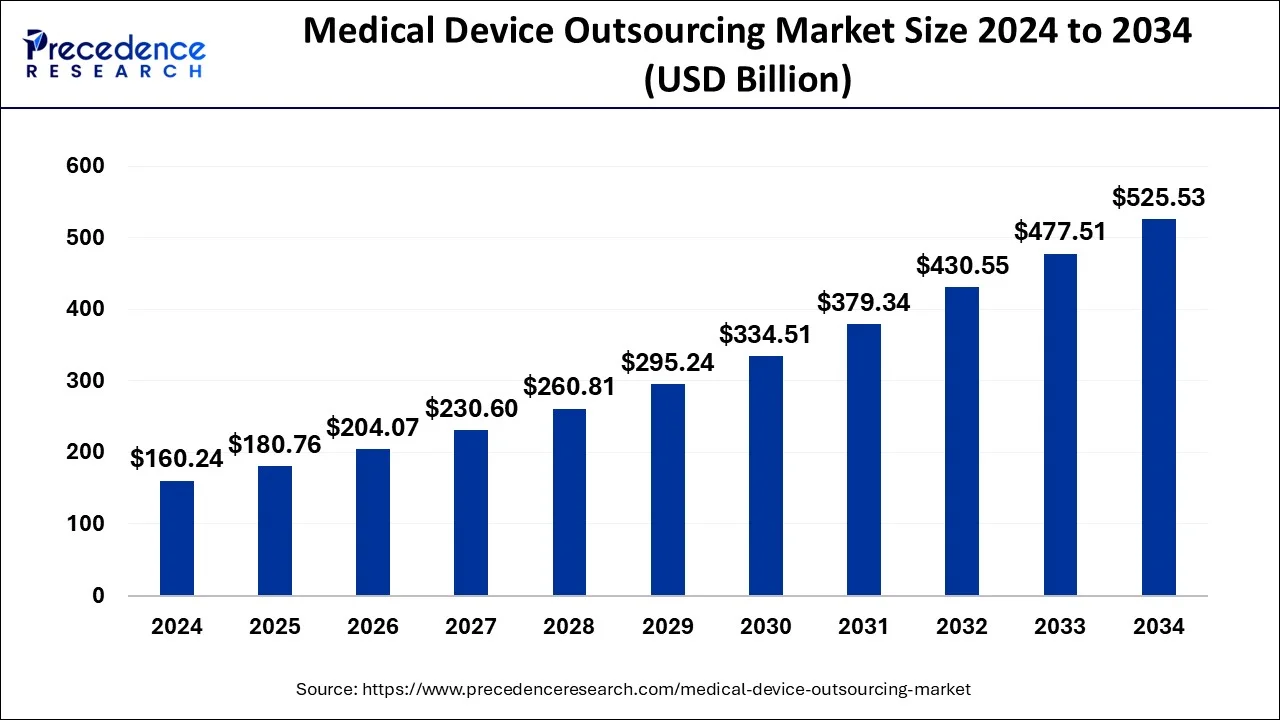
The global medical device outsourcing market size was calculated at USD 160.24 billion in 2024 and is anticipated to attain around USD 525.53 billion by 2034 with a CAGR of 12.61%.
Get Sample Copy of Report@ https://www.precedenceresearch.com/sample/1057
Key Points
- In 2024, Asia Pacific held the dominant position in the medical device outsourcing market with a 41.79% share.
- North America is set to grow at the most rapid CAGR of 12.5% throughout the forecast period.
- The cardiology segment registered the highest market share among applications in 2024.
- The quality assurance service segment led the market, contributing 9.56% of the total share in 2024.
- The regulatory affairs services segment is projected to expand significantly at a CAGR of 13.5% during the forecast period.
The Game-Changing Effects of AI on Medical Device Outsourcing
- AI-Powered Design Optimization – AI functions like a digital architect, refining medical device designs for optimal performance and compliance.
- Personalized Product Development – Just as AI personalizes online shopping, it enables customized medical device development based on patient-specific data.
- Enhanced Testing and Validation – AI simulates real-world scenarios, acting like a virtual test lab to ensure medical devices meet strict safety and performance standards.
- Streamlined Documentation and Reporting – AI automates regulatory documentation, much like an efficient secretary, reducing human error and speeding up approvals.
- Seamless Collaboration Across Borders – AI-powered cloud platforms facilitate real-time communication and data sharing between outsourcing partners worldwide, ensuring smoother coordination.
Market Scope
| Report Highlights | Details |
| Market Size in 2024 | USD 142.19 Billion |
| Market Size in 2025 | USD 142.19 Billion |
| Market Size by 2034 | USD 430.55 Billion |
| Growth Rate from 2025 to 2034 | CAGR of 13.10% |
| Largest Market | Asia Pacific |
| Base Year | 2024 |
| Forecast Period | 2025 to 2034 |
| Segments Covered | Service Type, Application Type, Region Type |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Market Dynamics
Market Drivers
The medical device outsourcing market is expanding due to rising healthcare demands, cost constraints, and the need for specialized expertise. Original equipment manufacturers (OEMs) are outsourcing production, regulatory compliance, and quality assurance to improve efficiency and meet growing market demands. Technological advancements, such as automation and AI-driven quality control, are further accelerating the adoption of outsourcing strategies. Additionally, stringent global regulatory frameworks are encouraging companies to rely on external regulatory affairs services to ensure compliance.
Market Opportunities
Opportunities in the market are emerging with the rise of precision medicine and personalized medical devices. The increasing adoption of 3D printing and AI in medical device manufacturing is enhancing production efficiency and customization capabilities. Expanding healthcare infrastructure in developing countries is also creating demand for outsourced medical device production, as companies seek cost-effective solutions. Moreover, the increasing role of contract research organizations (CROs) in clinical trials and product testing is further boosting outsourcing activities.
Market Challenges
Key challenges in the industry include regulatory complexities, as different regions have varying approval processes and compliance requirements. Protecting intellectual property rights is another critical concern, as outsourcing firms handle sensitive designs and production methods. Additionally, global supply chain disruptions, caused by factors such as raw material shortages and transportation delays, pose risks to timely production and delivery. Ensuring consistent product quality across different manufacturing facilities also remains a challenge for outsourcing companies.
Regional Insights
- Revenue Cycle Management Market Size to Attain USD 451.29 Bn by 2034 - March 5, 2025
- Life Science Tools Market Size to Attain USD 409.99 Bn by 2033 - March 5, 2025
- mHealth Apps Market Size to Attain USD 154.12 Billion by 2034 - March 5, 2025