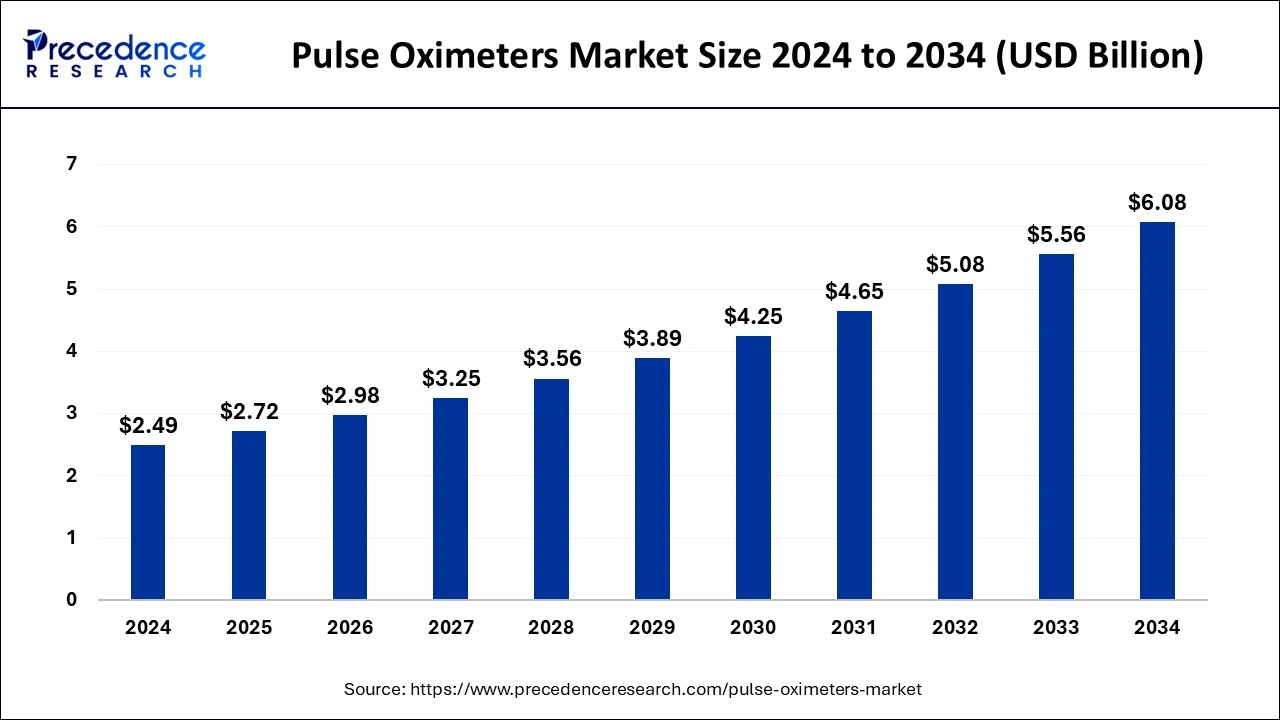
The pulse oximeters market size was calculated at USD 2.49 billion in 2024, and it is projected to be attain around USD 6.08 billion by 2034 with a CAGR of 9.33%.
Get Sample Copy of Report @ https://www.precedenceresearch.com/sample/1036
Key Insights
- In 2024, North America held the largest share of the market at 49%.
- The hospitals segment was the top contributor in the market by end-use.
- The home healthcare segment is expected to grow at the highest CAGR over the forecast period.
- Tabletop oximeters had the highest market share among product types in 2024.
The Role of AI in Transforming the Pulse Oximeters Market
The integration of artificial intelligence in pulse oximeters is enhancing their accuracy and reliability. AI-powered algorithms help in minimizing errors caused by skin pigmentation, motion artifacts, and ambient light interference.
Additionally, AI enables predictive analytics, allowing healthcare providers to detect early signs of respiratory distress or other complications. With continuous advancements, AI-driven pulse oximeters are becoming more effective in remote patient monitoring, telemedicine, and personalized healthcare solutions.
Market Drivers
The increasing prevalence of chronic respiratory conditions and the growing geriatric population are key drivers of the pulse oximeters market. The demand for non-invasive and portable monitoring devices has surged, especially after the COVID-19 pandemic highlighted the importance of tracking blood oxygen levels. The rising integration of artificial intelligence in healthcare is also driving innovation, leading to the development of more accurate and user-friendly pulse oximeters.
Opportunities
The shift towards digital healthcare and telemedicine presents immense opportunities for market expansion. Companies are investing in the development of wearable pulse oximeters that provide real-time monitoring and predictive analytics, improving patient outcomes.
The growing popularity of fitness and wellness devices also creates new avenues for market penetration, as more consumers seek health-tracking gadgets for daily use.
Challenges
One of the biggest challenges in the pulse oximeters market is the variability in accuracy across different skin tones and conditions.
Additionally, competition from low-cost alternatives and counterfeit products affects brand reputation and consumer confidence. Regulatory hurdles and compliance requirements remain a challenge for new market entrants, limiting the speed of innovation and product launches.
Regional Insights
North America remains a leading market for pulse oximeters, supported by technological advancements and well-established healthcare facilities. Europe is experiencing steady growth, driven by increasing investments in digital health solutions.
The Asia-Pacific region is witnessing rapid expansion due to a rising number of respiratory disease cases and improved healthcare access. Latin America and the Middle East & Africa are also emerging markets, with gradual adoption of home-based monitoring solutions and increased government healthcare spending.
Pulse Oximeters Market Companies
- Smiths Group plc.
- Halmaplc
- Koninklijke Philips N.V.
- Medtronic plc
- Nihon Kohden Corporation
- Masimo Corporation
- Contec Medical Systems Co., Ltd.
- Omron healthcare, Inc.
- General Electric Company
- Nonin Medical, Inc.
Leadership Announcement
Prevounce Health comes as one of the leading providers of software for remote care management by announcing its first blood oxygen device under the venture of remote patient monitoring (RPM): Pylo OX1-LTE. According to Daniel Tashnek, founder and Chief Executive Officer of Prevounce, this device was an exciting addition to the Pylo family.
Even as the device delivers actionable data to clinicians for empowering care, it will be allowed to be used much more easily by patients. This will ensure increased involvement and participation in RPM programs while reducing both clinician and technical support work needed to scale up remote monitoring programs.
Recent Developements
- In January 2025, the United States Food and Drug Administration released guidelines for manufacturers regarding the calibration of their devices to better read pulse oxygen readings for people of color, by ensuring devices are tested across a variety of skin tones. The new guidelines ask companies to mandate the collection of 3,000 data points rather than 200. Studies conducted using these meters will also need to be composed of a minimum of 10 people, with 150 being the recommended sample size.
- In August 2024, Prevounce Health, a leading medical device provider, launched a remote patient-monitoring blood oxygen measurement device called Pylo OX1-LTE. The device is cellular connected, offering secure data collection, reliability and improved connectivity. The device is cost-effective, making it easier for organizations to invest in growing their remote patient monitoring programs.
Segments Covered in the Report
By Product Type
- Handheld Oximeters
- Fingertip Oximeters
- TabletopOximeters
By End-user
- Ambulatory Surgical Centers
- Hospitals
- Home Healthcares
By Regional Outlook
- North America
- U.S.
- Canada
- Europe
- U.K.
- Germany
- France
- Asia Pacific
- China
- India
- Japan
- South Korea
- Rest of the World
Ready for more? Dive into the full experience on our website@ https://www.precedenceresearch.com/
- Transplantation Market Size To Surge USD 39.19 Bn By 2034 - March 4, 2025
- N95 Medical Protective Masks Market Size to Soar USD 8.25 billion by 2034 - March 4, 2025
- Disposable Endoscopes Market Size to Hit USD 10.82 Bn by 2034 - March 3, 2025